BACKGROUND
T cells engineered with a CD19-targeted chimeric antigen receptor (CD19 CAR T cells) have revolutionized the treatment of high-risk, relapsed, or refractory large B-cell lymphoma (LBCL), but durable responses are only achieved in 30-40% of patients (pts). Outcomes are dismal in case of disease recurrence after CD19 CAR T-therapy; in this situation we have previously reported low efficacy and limited duration of response after a second infusion of the same CD19 CAR T-cell product (Gauthier, Blood, 2022). We observed that repeat CD19 CAR T-cell infusions were impaired by CD8+ T-cell responses against murine amino acid sequences located in the single chain variable fragment (scFv) of the CAR, which led to rapid immune rejection of CAR T cells. We hypothesized that CAR T cells engineered with a fully human scFv could circumvent anti-murine scFv immune responses in pts previously exposed to murine scFv-bearing CAR T cells. We report our phase 2 clinical trial results investigating treatment with fully human scFv-bearing CD19 CAR T cells (JCAR021) in R/R LBCL pts with relapsed or progressive disease after murine scFv-bearing CD19 CAR T-cell therapy.
METHODS
Pts with R/R LBCL were enrolled at the Fred Hutchinson Cancer Center on a phase II study (NCT03103971). Key eligibility criteria included: i) relapsed disease after complete response (CR) to prior CD19-targeted non-JCAR021 CAR T-cell therapy or persistent disease after partial response to prior CD19-targeted non-JCAR021 CAR T-cell therapy; ii) CD19-positive disease since completing the prior CD19-targeted CAR T-cell therapy. Pts received lymphodepletion (LD) with cyclophosphamide 300 mg/m2/d and fludarabine 30 mg/m2/d for 3 days. Cytokine release syndrome (CRS) and neurotoxicity (NT) were graded according to the Lee 2014 criteria and CTCAE v4.03 criteria, respectively. Disease response was evaluated at day +28 after JCAR021 infusion per PET-CT Lugano criteria. The planned sample size and CAR T-cell dose were n=27 and 7x10 6 CAR T cells/kg, respectively.
RESULTS
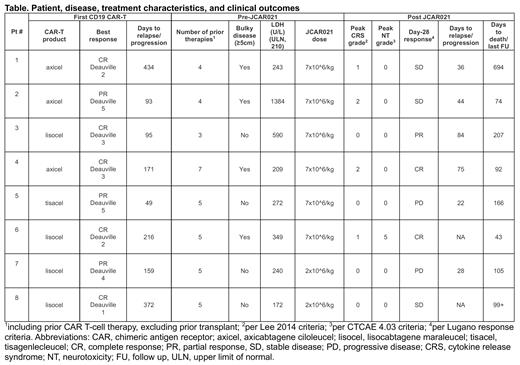
Pt characteristics, and outcomes are shown in the Table. All eight screened pts were enrolled, received LD, and JCAR021 infusion. Median age at enrollment was 63 (range: 41-77). Prior to lymphodepletion, 4 pts (50%) had bulky disease (largest lesion ≥5cm). All pts had extranodal disease. We observed elevated LDH prior to lymphodepletion in 6 pts (75%). Median prior lines of therapy was 5 (range: 3-7). Prior CD19 CAR T-cell product type was lisocabtagene maraleucel, n=4 (50%); axicabtagene ciloleucel, n= 3 (37%); or tisagenlecleucel, n=1. Response to the prior CD19 CAR T-cell therapy was CR in 5 pts (62%) and PR in 3 pts (38%). Median time from the first CAR T-cell infusion to relapse or progression was 165 days (range 49-434). Median time from the first CAR T-cell infusion to JCAR021 infusion was 304 days (range: 202-603). JCAR021 was administered at the dose of 7x10 6 (n=6, 75%) or 2x10 6 CAR T-cells/kg (n=2, 25%; dose reduction due to grade 5 NT in subject #6).
We observed all-grade CRS in 4 pts (50%; grade 1, n=2; grade 2, n=2 not requiring vasopressors) and NT in 1 patient (dose limiting toxicity, grade 5 NT from intracerebral hemorrhage with concurrent acute kidney injury and toxic encephalopathy).
At day+28 responses were seen in 3 of 8 pts (37%; CR, n=2; PR, n=1), all of whom received 7x10 6 CAR T-cells/kg. Two pts in CR after JCAR021 had bulky disease and elevated LDH prior to LD. In both pts, the prior CD19 CAR T-cell therapy had induced CR lasting ≥ 6 months. In the two evaluable responders (death in CR, n=1), we observed disease relapse or progression within 3 months. Median overall survival was 105 days (95%CI, 92-NA).
We measured expansion of JCAR021 CAR T-cells in all pts. Exploratory analyses showed lower in vitro CAR T-cell expansion during manufacturing (fold expansion of CD8+ CAR T-cells, p<0.001) and lower in vivo CAR T-cell expansion (peak CD8+ CAR-T, p=0.002; AUC day0-28, p=0.01; JCAR021 dose, 7x10 6/kg) compared to pts receiving JCAR021 as their first CD19 CAR-T product (CAR-naïve cohort).
CONCLUSION
In LBCL pts with disease relapse or progression after a first CD19 CAR T-cell therapy, we observed low response rates and lack of durable responses after treatment with the fully human scFv-bearing product JCAR021, prompting early study termination. Lower CAR T-cell expansion during manufacturing in CAR-exposed pts suggest pre-existing T-cell dysfunction as a potential mechanism of failure.
Disclosures
Gauthier:Century Therapeutics: Other: Independent data review committee; Angiocrine Bioscience: Research Funding; Sobi: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Research Funding; Juno Therapeutics (a Bristol Myers Squibb company): Research Funding; Kite Pharma: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene (a Bristol Myers Squibb company): Research Funding; Legend Biotech: Consultancy, Honoraria. Shadman:MorphoSys/Incyte: Consultancy, Research Funding; MEI Pharma: Consultancy; Kite, a Gilead Company: Consultancy; Mustang Bio: Consultancy, Research Funding; Vincerx: Research Funding; Genmab: Consultancy, Research Funding; TG Therapeutics: Research Funding; ADC therapeutics: Consultancy; Pharmacyclics: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Fate Therapeutics: Consultancy; Eli Lilly: Consultancy; Regeneron: Consultancy; Janssen: Consultancy; AbbVie: Consultancy, Research Funding. Hirayama:Juno Therapeutics, a Bristol Myers Squibb Company: Research Funding; Novartis: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Nektar Therapeutics: Honoraria, Research Funding. Till:BMS/Juno Therapeutics: Research Funding; Mustang Bio: Consultancy, Patents & Royalties, Research Funding; Proteios Technology: Consultancy, Current holder of stock options in a privately-held company. Kimble:Juno/BMS: Research Funding. Iovino:Mustang Bio: Current equity holder in publicly-traded company. Chapuis:Juno Therapeutics: Research Funding. Cassaday:Autolus: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; PeproMene Bio: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Vanda Pharmaceuticals: Research Funding; Servier: Research Funding; Jazz: Consultancy, Honoraria; Seagen: Other: Spouse was employed by and owned stock in Seagen within the last 24 months.. Milano:ExCellThera Inc.: Research Funding. Poh:Acrotech: Consultancy; Incyte: Research Funding; Seattle Genetics: Consultancy; BeiGene: Consultancy. Gopal:Compliment Corporation: Current holder of stock options in a privately-held company; Incyte, Kite, Morphosys/Incyte, ADCT, Acrotech, Merck, Karyopharm, Servier, Beigene, Cellectar, Janssen, SeaGen, Epizyme, I-Mab bio, Gilead, Genentech, Lilly, Caribou, Fresenius-Kabi: Consultancy; Merck, I-Mab bio, IgM Bio, Takeda, Gilead, Astra-Zeneca, Agios, Janssen, BMS, SeaGen, Teva, Genmab: Research Funding. Riddell:Juno Therapeutics: Consultancy, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Other: Co-founder, Research Funding; Lyell Immunopharma: Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Other: Co-founder, Patents & Royalties, Research Funding; Adaptive Biotechnologies: Consultancy; Ozette Technologies: Membership on an entity's Board of Directors or advisory committees. Maloney:A2 Biotherapeutics: Consultancy, Current holder of stock options in a privately-held company, Honoraria, Other: Member of the Scientific Advisory Board; Amgen: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Other: Member of the JCAR017 EAP-001 Safety Review Committee and Member, CLL Strategic Council, Member of the JCAR017-BCM-03 Scientific Steering Committee under BMS, Research Funding; Novartis: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board , Research Funding; Genentech: Consultancy, Honoraria, Other: Chair and Member of the Lymphoma Steering Committee; Kite, a Gilead Sciences: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Juno Therapeutics: Consultancy, Honoraria, Patents & Royalties: Rights to royalties from Fred Hutch for patents licensed to Juno Therapeutics/BMS, Research Funding; Pharmacyclics: Consultancy, Honoraria; Umoja: Consultancy, Honoraria; Bioline Rx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Participation on a Data Safety Monitory Board ; Fred Hutch: Other: rights to royalties for patents licensed to Juno; Janssen: Consultancy, Honoraria; Legend Biotech: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria, Other: Member, Scientific Review Committee, Research Scholars Program in Hematologic Malignancies; Navan Technologies: Consultancy, Honoraria, Other: Member of the Scientific Advisory Board; Mustang Bio: Consultancy, Honoraria; Navan Technologies: Current holder of stock options in a privately-held company; Chimeric Therapeutics: Other: Member of the Scientific Advisory Board; ImmPACT Bio: Other: Member, Clinical Advisory Board, CD19/CD20 bi-specific CAR-T Cell Therapy Program; Interius: Other: Member, Clinical Advisory Board; Lyell Immunopharma: Other: Member, CAR T Steering Committee. Turtle:Kyverna: Other: DSMB Member; Juno Therapeutics/BMS, Nektar Therapeutics: Research Funding; Eureka Therapeutics, Caribou Biosciences, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics: Other: Stock options; CJT has the right to receive payment from Fred Hutch as an inventor on patents related to CAR T-cell therapy: Other: Patents; Caribou Biosciences, T-CURX, Myeloid Therapeutics, ArsenalBio, Cargo Therapeutics: Membership on an entity's Board of Directors or advisory committees; Nektar Therapeutics, Century Therapeutics, Legend Biotech, Allogene, Sobi, Syncopation Life Sciences, Prescient Therapeutics: Other: Ad hoc advisory boards/consulting (last 12 months).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal